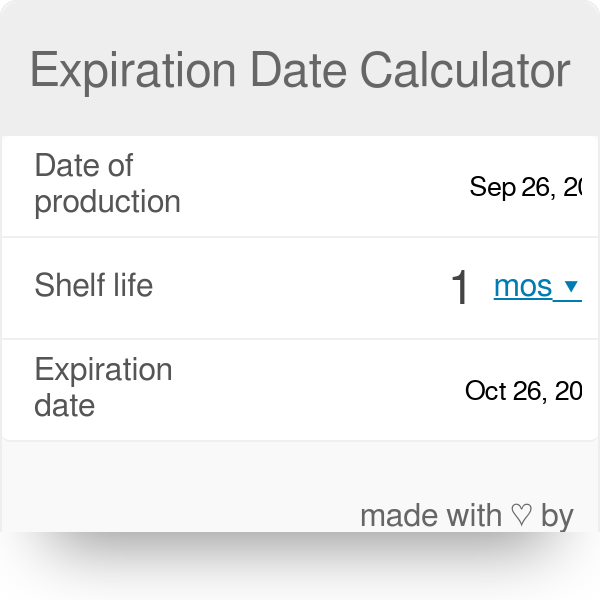
shelf life calculator for pharmaceutical products
Click Calculate at the bottom of the calculator to determine AAT rounded up to the nearest day. How do you calculate remaining shelf life.

How Can The Pharmaceutical Sector Reduce Its Carbon Footprint Pharmaceutical Engineering
Absence of an Expiration Date.
. Shelf Life Calculator For Composites And Other Materials Shelf Life Of Foods First Order Kinetics Example Youtube Evaluation Of Shelf Life Of Drug Products By Arrhenius Equation Part I Youtube 2 Calculation Of Expiry Date Shelf Life Of Medicine By Accelerated Stability Study Method In English Youtube Microsoft Excel Shelf Life Calculate On Which Day Left Equals 75. If youve not already done so complete the short form to see the result. Input the Aging Factor Q10 value for your product.
The shelf life time t with a minimum label claim of 95 can be calculated by replacing 095D 0 D t in equation 12. 3M state All 3MVHBTapes have a shelf life of 24 months from date of manufacture when stored at 4C to 38C and 0-95 relative humidity. Establishing the Shelf Life of Pharmaceutical Products.
The default Q10 value is set at 2. What should be the accelerated stability testing and shelf-life calculation is explained in the 21 CFR part 211137- Expiration dating. Input the Aging Factor Q10 value for your product.
The optimum storage conditions are 22C and 50 relative humidity. 1996 Statistical evaluation of shelf-life of pharmaceutical products estimated matrixing Drug Stab. The shelf life of a product may vary between different countriesregions depending on regulatory requirements.
For example the shelf life of a product at 50 C is 32 days. Accelerated stability testing and shelf-life calculation. Input the TAA and TRT values both in Celsius for your product.
At that time Q10n 3 25 156. Normal storage temperature is 25 C. This guideline applies to human and veterinary medicines.
Arguments are presented that for regulatory and statistical reasons the true product shelf life should be defined in terms of a suitably small quantile eg fifth of the distribution of batch shelf lives. This document assists with establishing the expiration period of a production bath of a medicinal productIt is not applicable to biological medicinal products such as vaccines sera toxins and allergens products derived from human blood and plasma as well as medicinal products prepared biotechnologically. Each batch is distinguished by its own shelf life which can be called the true batch shelf life.
The higher the Q10 the longer the expiry date. Input the TAA and TRT values both in Celsius for your product. Thus the true shelf-life denoted by θ is the solution of η α βx hence θ η α β Notethat α is the average drug characteristic at the time of manufacture ie x 0 which is usually larger than η Thus θ 0.
The batch is a single sample of the pharmaceutical products manufacturing process at. The absence of an expiration date on any drug product packaged after September 29 1979 except for those drugs specifically exempt by. The choice of quantile translates to an upper bound on the probability that a randomly selected batch will be nonconforming when tested at.
Then you may use the formula 025B2075A2 in C2 on my sample please remember to set the cells with results as Date. 32 days x 156 500 days. Better estimates of product shelf life 378 months disincentive for industry to include more stability batches Pharmaceutical Stability Shelf Life August 1 2010 20 3-Batch Estimate of Shelf Life n 466 18 mean 229 months SD 586 Comparison of ICH Shelf Life Estimation Methodology Using Industry Data.
As a result of the publication of 21 CFR Part 211 Current Good Manufacturing Practice for Finished Pharmaceuticals requirements were outlined concerning the expiration. Expected shelf life is usually. The fact it can cope equally well in total opposite temperatures and conditions means the storage conditions have to be spot on.
For example A manufacturing date B expiry date C Date at 75 remaining. The quality within each step influences the quality of the resulting knowledge. With the USP acceptance criteria of 100 15 from USP.
At least three distinct steps are necessary to gain knowledge and understanding of a product or process. Therefore developing and instituting best scientific methods at each step supports the ongoing Quality-by. On the Shelf Life of Pharmaceutical Products.
The shelf life of the pharmaceutical products is the time period for which the product maintains its identity and quality when stored at the conditions defined on the label of the product. It is used to simulate real shelf-life aging and is conducted to validate shelf-life claims and document expiration dates. For example the shelf life of a product at 50 C is 32 days.
A pharmaceutical product is typically manufactured in batches. Accelerated aging studies can be used for shelf life determination but must later. Which equation is used for predicting the shelf life of a drug product.
The labeled shelf life is what is printed on the drug products label and is used to calculate the expiry date. The shelf life for a combination product is determined from drug stability device aging and sterile barrier aging with the shortest estimate determining the overall shelf life. A pharmaceutical product is typically manufactured in batches.
N 50-25 10 25. QUANTILE REGRESSION CALIBRATION. A batch is a fixed quantity of product for example 100000 tablets.
Batch and Product Shelf Life. Suppose Q10 3.

Shelf Life Calculator For Composites And Other Materials

Loss Of Exclusivity Strategies To Maximize Product Value

Microsoft Excel Shelf Life Calculate On Which Day Left Equals 75 Super User

Shelf Life Calculator For Composites And Other Materials

Shelf Life Calculator For Composites And Other Materials
Adderall Vs Modafinil How Do They Compare

Margin Of Safety Calculation Formula What Is Margin Of Safety In Pharmacology Video Lesson Transcript Study Com

Shelf Life Calculator For Composites And Other Materials

Shelf Life Calculator For Composites And Other Materials

Fda Approves Drug To Treat Dangerously Low Blood Pressure

Office World 252xl Ink Cartridge Magenta New Sealed Officeworld Ink Cartridge Ink Cartridges

Shelf Life Calculator For Composites And Other Materials

Specialty Pharmacy The Complete Pharmacist Specialty Pharmacy Pharmacy National Cancer Institute

Shelf Life Calculation Of Drugs
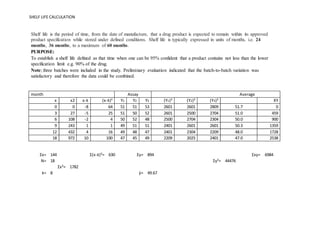
Shelf Life Calculation Of Drugs

Drug Half Life Explained Calculator Variables Examples